St Anne’s Academic Review 8 – 2018
Meat without the animals: cleaning our conscience with clean meat
Alex Norman and Pranay Shah, Department of Biochemistry
STAAR 8 – October 2018, pp. 48-65
——————————–
Published: 03 Oct 2018
Review process: Open Peer Review
Draft First Uploaded: 16 Jul 2018. See draft and reviewers’ comments.
Abstract
Clean meat is meat made by the process of culturing animal stem cells in such a way that they produce muscle tissue. The technology reproduces the 3D structure of muscle fibres, closely replicating those found in conventional meat, but without requiring factory farming or slaughtering of animals. Many consider it as a viable option to sustain the world’s growing population, on both nutritional and environmental fronts. Although the technology is still in development, increased resources and funding for clean meat research have led to notable advances made possible by scientists in academia as well as in start-ups funded by the likes of Richard Branson and Bill Gates. Still, as the field moves forward, two areas need to be addressed for clean meat to successfully disrupt the current meat industry: first is the perception of lab-grown meat by the public and governments, particularly regarding consumer uptake, regulation and legislation. In addition, technical challenges still remain, including the up-scaling of production to commercial levels and the engineering of more complex cellular structures to better replicate the taste, consistency and texture of meat. In this review, we will discuss the background for clean meat development, and the issues it is trying to address, followed by the production methods. We end with a discussion of current obstacles and our proposals for the future of clean meat.
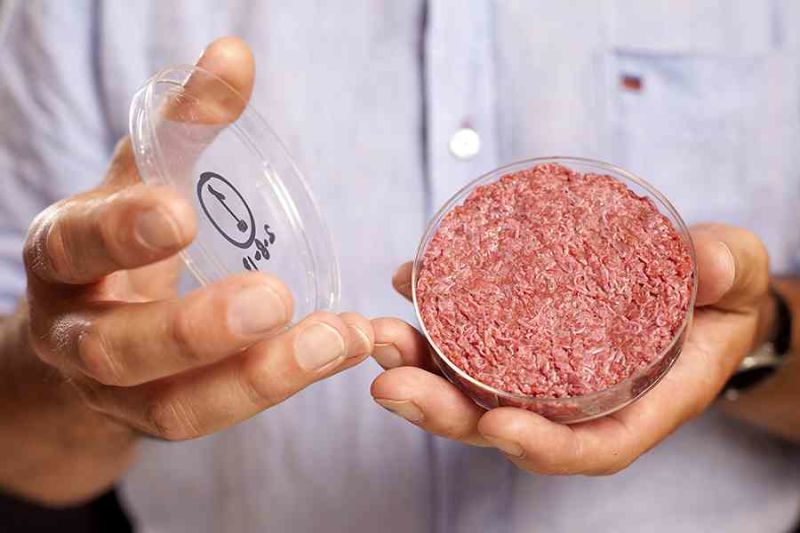
Introduction
“We shall escape the absurdity of growing a whole chicken in order to eat the breast or wing, by growing these parts separately under a suitable medium.” wrote Winston Churchill in his 1932 essay Fifty Years Hence. Despite being acknowledged over 85 years ago, this absurdity still remains. There have been attempts to realise Churchill’s vision using meat alternatives based on alternative protein sources such as plants, but in the opinion of many, they fall short of accurately mimicking meat in all its sensations, from sight and feel to taste. Now, decades of biochemical research in tissue engineering, cell culture and protein science amongst other disciplines, are being brought together in the ground-breaking field of clean meat.
This was showcased to the world for the first time on the 5th of August 2013 when well-renowned chef Richard McGeown cooked live on air a hamburger made entirely from cultured stem cells. It was the culmination of several years of work in the laboratory of Dr Mark Post, a Dutch cardiologist turned food scientist who has since founded a start-up developing clean meat. His work had constructed the burger in a laboratory by differentiating and growing stem cells derived from cows.
This was no small feat: the technologies necessary to grow animal tissue were made possible by the preceding decades of stem cell and tissue research. This had not been conducted with the goal of growing muscle for human consumption but primarily in the field of regenerative medicine. However, Dr Post, formerly an assistant professor of Medicine at Harvard Medical School, realised that this research could be used to develop a new avenue of food technology and he pivoted into the fields of vascular physiology and tissue engineering, moving to the Netherlands to establish research groups in these areas.
That world-first clean meat burger from his research group cost over $300,000 to produce and was funded in part by Google-cofounder Sergey Brin [1]. Since then, when the goal was a proof-of-concept example of meat grown in the laboratory that was fit for human consumption, the cost of clean meat has gone down to $5,280/kg as of June 2017 [2].
In the process of clean meat production, living animals are initially used to donate muscle stem cells. Due to the exponential growth of cells in optimum conditions, only a single isolation would theoretically be needed for unlimited meat production and so animals would not be required beyond this first stage. As a result, the meat is ‘clean’ since it is free from the conditions animals face in factory farms, free from animal slaughter and also free from the antibiotics fed to animals farmed for food.
In this piece, we begin by describing the ‘absurdity’ that clean meat is tackling from the environmental to ethical issues. We then describe how scientists, NGOs, for-profit companies and governments are working together to realise the mass production of clean meat. Finally, we outline the obstacles that the field of clean meat currently faces and give our views on its future directions, including the important issue of public acceptance on shifting the production of animal meat from living animals to bioreactors in food factories.
The foundations and reasons for pursuing clean meat
Clean meat technology intends to supersede current livestock farming, an industry that generates a substantial burden on the planet and which is set to become economically unfeasible. Below we outline some of the current problems that arise from animal agriculture:
Economics and Sustainability
Global meat consumption has risen substantially in recent years, increasing approximately 5-fold in the 50 years between 1960 and 2010 [3]. This increase in meat consumption has been a differential one: high income countries in the West have maintained roughly comparable meat consumption levels in the last 30 years, whilst there has been a roughly 3-fold increase in meat consumption in China, as well as notable increases in East Asian middle-income countries [3]. Overall, global meat consumption has doubled in the last 30 years alone [3] due to nations switching from a reliance on grains and starches to vegetables, fruits and meat as wealth increases [4]. Economically, this is partly due to a fall in the relative price of meat as a country’s wealth increases although a number of social and cultural factors play a role too [5].
The implications of a rising global population, projected to be nearly 10 billion by mid-century [6], combined with increasing global economic growth are clear: unless supply scales with demand, there will be a significant mismatch between the amount of meat we can produce and its global demand [7, 8]. Although several projections of global meat consumption, using different methods, calculate different figures for the 2050 level [9-11], they all agree that the increase will be significant. Since some estimates even put forward that livestock meat production is already at its upper limit [7], there is a case to be made that meat is on track to become a luxury commodity affordable to a wealthy minority [8].
Environmental Burden
On top of the looming economic issues, a second set of problems associated with the agricultural livestock industry is the burden on the environment, including greenhouse gases, water and land use.
Greenhouse gases
Greenhouse gases (GHGs) like CO2, methane and N2O are well-established contributors to climate change [12]. They are produced at different stages and to varying degrees by livestock farming [13] for example, N2O is produced from manure and soil whereas methane is produced from both enteric fermentation and manure. In particular, cattle bred for meat production are substantial contributors to methane emission and are estimated to contribute around 50 million tonnes of methane per year. This is over three times the quantity of methane produced by the next largest methane-producing livestock, dairy cattle, and over 60% of total methane emissions from livestock enteric fermentation [14].
Estimates of the percentage of total GHG emissions caused by animal agriculture range from 8 – 18% [15]. The Food and Agriculture Organisation of the United Nations estimates that 14.5% of total anthropogenic GHGs are produced from livestock farming [14].
Land and Energy Use
Livestock land use comprises 80% of total agricultural land, with most of this in turn being made up of grazing based pasture [16]. Amazingly, this vast amount of land ultimately produces 1% of global edible energy [16] due to the energy losses at each trophic level. One measure of energy efficiency of livestock production is to divide the calorific energy contained in the meat product by the total calorific energy in the animal’s feed (this is called the feed efficiency). While this is a useful and intuitive estimate there is also much uncertainty in its calculation. For example, beef efficiency estimates vary widely between studies [16]. The different regional values obtained for this metric from different studies reflect factors such as incomplete data and alternative regional definitions [16].
The main contributing factor to the low feed efficiency is the prolonged reproductive rate of livestock like beef and sheep with the least feed-efficient livestock being cattle, whose efficiency estimates range from 0.3 – 2% [16].
Water Use
Livestock farming also significantly relies on water resources. Mekonnen and Haekstra have carried out analyses into the global water footprint of meat livestock farming [17]. They calculate that for every animal product there is a larger water footprint than the calorie equivalent of any crop [17]. For example, beef has 20 times the water footprint per calorie than cereals, which again is a consequence of the low feed efficiency of animal products [17].
Clean meat – a solution?
Relatively few studies have directly attempted to quantify the environmental impact of clean meat production compared to livestock agriculture. The first notable attempt to do this was in 2011 by Hanna Tuomisto at the University of Oxford [18]. The study found that the projected energy cost to produce 1000 kg of clean meat would require 26 to 33 GJ energy. This figure is between 7 to 45% lower than the conventional livestock energy consumption [18].
However, a key assumption in this paper’s methodology was to omit the requirement of a constant energy input to heat the bioreactors in which cells grow [19]. In fact, the main energy cost to clean meat production is heating bioreactors, so this paper underestimated the energy use of clean meat production. A revised version of the study was published in 2014 [20], showing that the energy use of clean meat is on par with that of cattle (the most energy intensive livestock farming.). However, there is high uncertainty in these estimates [20].
Mattick and others also completed a projected life cycle analysis for clean meat based on mammalian cell culture lines and found even higher expected values for the energy consumption and land use [21]. Most of the energy for clean meat production is expected to be in the industrial processes of basal media production and bioreactor maintenance [21]. Given that these processes do not currently exist on a large scale, the estimates for energy usage have a large variance and there is considerable uncertainty associated with them. One way in which clean meat production could massively reduce emissions is if clean meat plants are located locally in the cities that they supply. This would reduce transport costs and the associated emissions, and potentially reduce land use if a vertical production system is employed.
Human Welfare
Finally, there are also consequences to human welfare that arise from modern day factory farming. The greatest concerns are the use of growth-promoting hormones and the gross overuse of antibiotics. Eighty percent of antibiotics used in the USA are given to livestock [22] and the inevitable development and proliferation of antibiotic resistance is rapidly manifesting as a global catastrophe. Furthermore, the incredibly densely populated spaces within factory farms are a major repository of potential human pathogens. Given that an estimated 60% of all human infectious pathogens are zoonotic in nature, this dense population represents an unimaginable public health concern [23]. Slaughterhouses and the production process notoriously lack adequate hygiene regulations, and several audits have revealed that meat contaminated with bacteria-containing fecal matter are able to get to market [24]. In this respect, clean meat offers an alternative because the conditions of a laboratory-based facility will be fully sterilised and there will be no need for antibiotic use. Moreover, few contagious pathogens that pose a public health threat infect tissue or muscle cells themselves, so in theory the pool of cells used for clean meat will have a reduced capacity as a repository for potential human disease-causing agents.
Clean meat in 2018 – what, how and who?
The production of clean meat is seen by many as a necessary development for there to be sustainable food production in the future. By being able to grow meat from cells, the issues mentioned above can be addressed without altering current eating habits, as the overriding aim of the clean meat field is to replace conventionally produced meat and match the price of the cheapest available meat, with possibly being even cheaper [25].
Although clean meat technology has been developed relatively recently, other meat alternatives have been commercially produced already. The science behind these products does not require the production of animal tissue from cells but the use of non-meat-based analogues to replicate the texture and taste of meat. Hence, whilst the production of these currently available meat substitutes relies on mimicking meat’s flavour and texture with alternative sources such as fungi, e.g. Quorn, or plant-based proteins, e.g. the Impossible Burger, clean meat aims to grow muscle tissue from cells that make up living animals, outside of the animal.
Production of clean meat
As clean meat utilises animal cells to grow tissue, it Is theoretically identical to conventional meat from animals. The work of Dr Mark Post mentioned earlier is an example of so-called cellular methods of clean meat production. There are now around 18 start-ups using similar cell-based techniques [26].
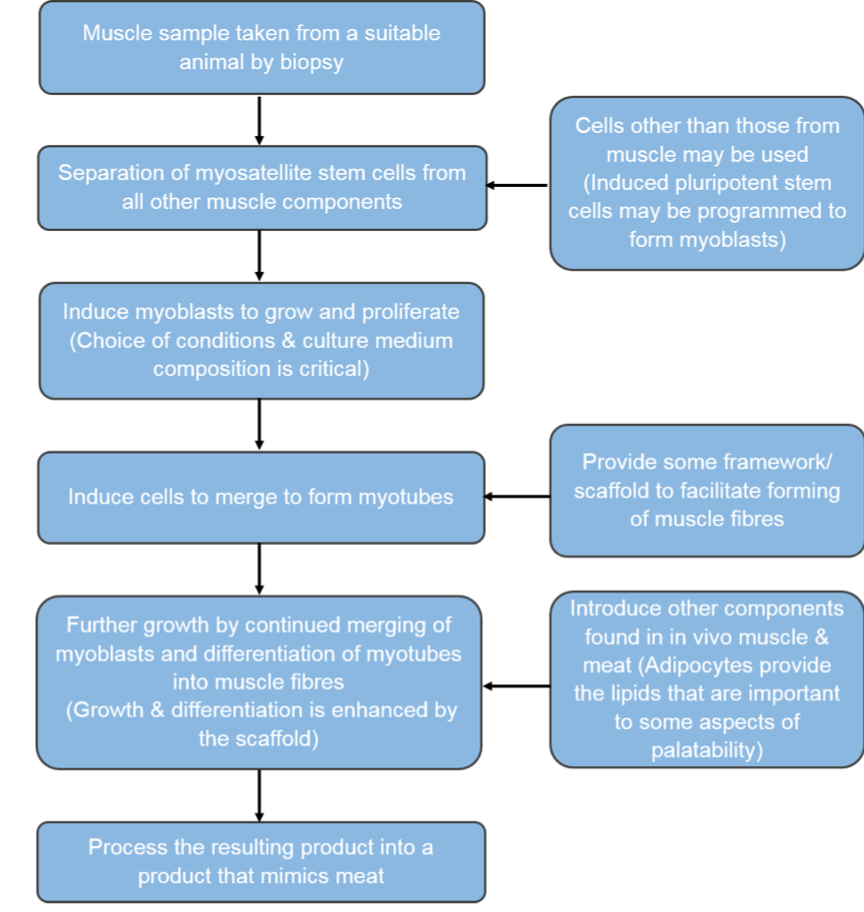
In a living organism (in vivo), the creation of muscle is the first step in the meat production process. In order to produce skeletal muscle in adult organisms, a type of stem cell called a myosatellite cell is activated in response to cues such as injury [27]. Because myosatellite cells are adult stem cells, they can only differentiate into a limited range of cell types that make up skeletal muscle [27]. Upon activation in vivo, the myosatellite stem cells initially differentiate into myoblast stem cells, which then transform into myocytes. Following this, the myocytes fuse to form myotubes and, in living animals, the myotubes align to make myofibrils that form muscle fibres and then fibre bundles and ultimately muscle [27].
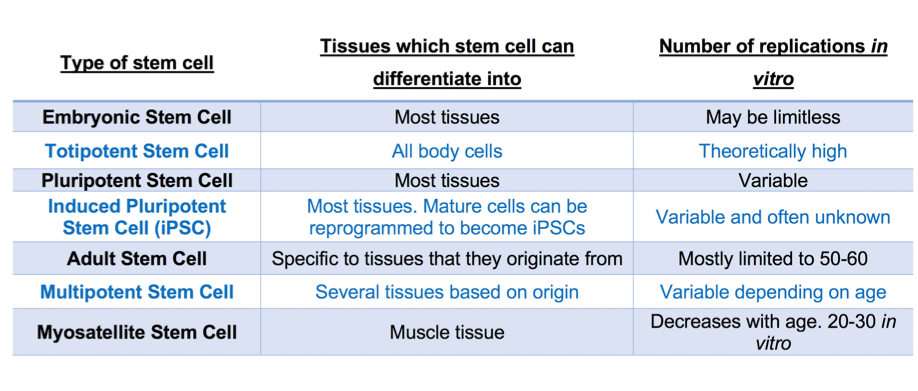
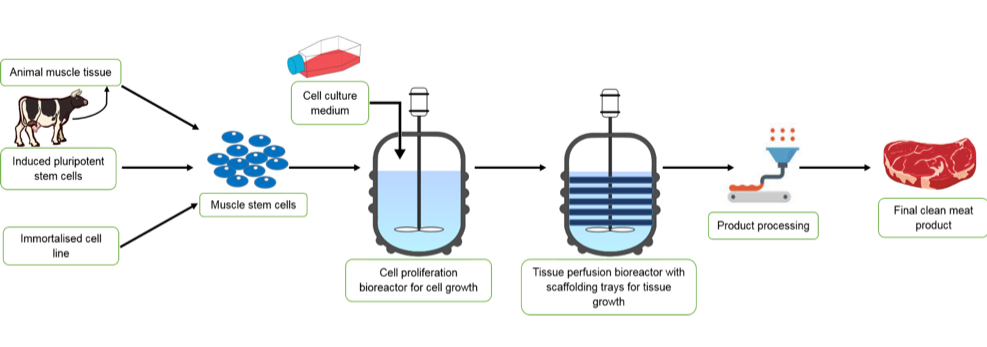
In clean meat research, the possibility of using several cell types to start production has been explored. This is because, as shown in table 1, the number of times a stem cell can replicate depends on the type of stem cell it is. The use of myosatellite cells to mimic in vivo muscle generation requires a minimally invasive muscle biopsy [28] to obtain a sample of muscle from which myosatellite stem cells are extracted. Satellite cells can divide up to around a maximum of 40 times in vitro, although 20-30 is practically more accurate [29]. As a burger patty probably contains around tens of billions of cells [29], alternatives to satellite cells are desirable and have been investigated. Induced pluripotent stem cells (iPSCs) are stem cells that are produced when mature cells are dedifferentiated into pluripotent states. During reprogramming into iPSCs, cells also become immortal and hence can divide for longer than satellite cells [30]. This would allow for fewer biopsies from animals, as a large number of cells could be generated from the initial population. Another possible route is to generate immortalised satellite cell lines using gene editing techniques to create satellite cells that can divide indefinitely. This would remove the need for biopsies at all, and also ensure consistency between clean meat companies as they could all use the same stock of cell lines. This process has been demonstrated by scientists at North Carolina State University who successfully grew a small turkey nugget in two weeks from the time of thawing an immortal cell line they had created [31].
During clean meat production, either of the three cell types described above are used to generate myoblasts in vitro, which are then allowed to self-renew, but not differentiate into myocytes. Once a sufficient number of myoblasts are obtained, they are transferred to new conditions which allow differentiation. This capacity of self-renewal and differentiation can be controlled by the components present in the cell culture medium. This usually contains nutrients such as vitamins, minerals and amino acids as well as serum which provides additional components such as hormones, growth factors and other cell survival and growth promoting factors. Serum is used as it is an easy way to obtain all the factors that most cells need to grow and divide in culture. Foetal bovine serum (FBS) – i.e. the serum from new-born calves – is most commonly used. The depletion of nutrients and serum starvation in the myoblast culture is what elicits their differentiation into myocytes and the subsequent formation of myotubes through myocyte fusion.
During clean meat production, the generation of muscle fibres from myotubes is directed into a 3D structure, to resemble conventional meat cuts, by scaffolds that promote myotube attachment. This is necessary as the scaffolds allow for nutrients to access all cells in the tissue and also provide structure for the product to grow and take shape on. In addition, mechanical stimulation of muscle in vivo influences the organisation, and hence texture, of conventional meat. In clean meat production, chemical stimulation through calcium exposure can mimic this by causing muscle contraction. Alternatively, mechanical stimulation by applying electric impulses during cell development, can also be used to augment muscle fibre growth [30]. Scaffolds play a role in this process, which is achieved in vivo by blood vessels and the extracellular matrix that surrounds cells and tissues. Further research is also ongoing to identify additional components needed for muscle tissue production in vivo, such as fat-providing cells (adipocytes) [32]. This is firstly being done so that conventional meat can be accurately mimicked and also to investigate how co-culturing cells (e.g. myoblasts and adipocytes) affects the make-up, and therefore nutritional value, of clean-meat products.
Two significant advantages of clean meat can be highlighted from the discussed scientific aspects. Firstly, the ability to grow cells in sterile culture conditions theoretically allows for the removal of antibiotic use in food production, as claimed by Memphis Meats CEO Uma Valeti [17]. Secondly, the control over which nutrients, e.g. vitamins and minerals, are added to the cell culture, and also which cells are co-cultured, allows control over the content of fat, protein and other biological molecules produced in the clean-meat product [16]. This creates the potential for cultured meat to be nutritionally tailored as it could be possible, for example, to decrease the content of cholesterol and increase the protein content.
The current commercial climate of clean meat research
The scientific developments, breakthroughs and funding for clean meat production have come from a vast array of organisations, reflecting the notably wide reach of clean meat in academia, industry and policy.
Clean meat research is taking place most prominently in the laboratories of start-ups such as Memphis Meats and Finless Foods. Memphis Meats, which revealed the world’s first cell-based meatball in 2016, is working on various products, such as clean chicken and clean duck and has announced product release in 2021 [34]. To date, they have raised over $20 million in funding from sources including Bill Gates, Richard Branson and, in 2018, food giant Tyson Foods [35]. Finless Foods is focused on producing cell-based fish meat using stem cells derived from fish tissue [36]. Their aim is to bypass current fish farming methods to make clean fish products that are healthier, cheaper, more environmentally friendly and produced with more sustainable methods than the aquaculture methods used today. In 2017 they demonstrated the first clean-fish fish cakes and have raised funding of $3.5 million in 2018 to develop cell-based bluefin tuna [37].
These start-ups are developed and funded through incubators, investment funds and private investors, showing how the clean meat field has taken advantage of global interest in start-ups. This has been accelerated through a shift in the focus of incubators from the traditional technology-based start-ups to ventures founded on deep science – i.e. based on discoveries from novel scientific research or research across many scientific disciplines. An example is IndieBio, a US-based incubator that is tailored to the biological sciences and to scientists that want to commercialise their research. They provide funding of $250,000 as well as laboratory space to help scientists create viable products from their initial research in the space of four months [38]. Finless Foods and Memphis Meats are two examples of clean meat companies that have gone through the IndieBio accelerator programme.
Alongside this however, scientific research into clean meat is also undertaken and supported by institutions such as NGOs, universities and governments. The Good Food Institute (GFI) is an NGO whose mission is “to build a healthy, humane, and sustainable food system” [39]. They do this through working with companies such as the start-ups mentioned earlier as well as with policy making institutions, academics, food stores and grant-making institutions [39]. One aim of this is to bring together non-profit areas, such as academic research, with companies requiring additional scientific expertise and research. In 2017 alone, they provided support to over 100 entrepreneurs working in cell- or plant-based meat [40]. An example of its success is the creation of a food firm in China, Dao Foods International Inc., which was formed through collaborative efforts of three venture groups brought together by the GFI [41]. Dao Foods’ aim is to bring clean meat to the Chinese market as the government seeks to cut the country’s meat consumption [42]. Moreover, by educating the institutions that provide research grants and make policy, they aim to create a supportive market for clean meat products both in terms of the funding available and the legislation.
The Oxford Martin School is another example of an institution that is bringing together different sectors to use interdisciplinary research to tackle global problems. As stated on its website, the school acknowledges food production and sustainability as a great global challenge of this century: “Without radical change to the way we produce and consume food […] there is a substantial risk of significant increases in food prices with major political, environmental and humanitarian consequences.” [43]. Its Future of Food research programme brings together the private sector, academia and government to help try and find solutions to the global food crisis. One aspect of the programme is the development of clean meat production. Other academic institutions also undertaking similar approaches to the Martin School include schools at Harvard, Tufts, Bath, Ottawa, Technion, and Maastricht, showing that the role of academia in bridging different disciplines and sectors is increasingly crucial on large-scale, global issues. Furthermore, the interdisciplinary approach undertaken by NGOs and academic schools is extremely important because, as outlined in the obstacles section below, the hurdles which are facing clean meat now, and those it is predicted to face in the future, are not only scientific.
In addition to these institutions, governments have also played a role in supporting clean meat research. In 2017 for example, the Chinese government signed a $300 million trade agreement with Israel that involved supporting three Israeli clean meat start-ups in order to support their introduction into China [25]. This highly significant deal was possibly the sign of a global shift in the role of governments in food production as they change from initiating and supporting direct research to investing in private companies.
Obstacles to the production and adoption of clean meat
There are three main technological barriers presently withholding the large-scale production of clean meat at a marketable price: the cell culture medium, the scaffold on which myotubes fuse into fibres and the upscaling of the production process [44].
Firstly, the culture medium in which cells are grown for clean meat will need to be serum free. This is because serum production requires animal slaughter, which clean meat is trying to eliminate. In addition, the composition of animal derived serum varies hugely between batches based on seasonal and geographical changes [45]. Furthermore, whilst demand for serum in research has increased, its supply has decreased leading to a price increase of 300% in the last few years [46]. In addition to this, its supply is not consistent as it is dependent on demand for meat such as beef (serum is a by-product of meat industries) as well as the availability of livestock, which fluctuates based on factors such as natural disasters [45]. Therefore, clean meat production requires a serum-free medium that will be cost-effective, reproducible and free from animal slaughter. Ideally this would involve using nutrients, e.g. growth factors and vitamins, made synthetically or using genetically modified organisms such as yeast. These would be used to create the optimal media for the different cell types that will be used in clean meat production such as iPSCs and myosatellite cells from different animals. There are already developments being made in this field with serum-free media having been shown to support muscle growth in vitro from sheep skeletal muscle stem cells [47] and also porcine iPSCs [48].
Another hurdle is the development of the scaffolds that the cells are grown on. These must allow cell adhesion to their surface and support the oxygenation and perfusion of media through the tissue either directly or through a vascular network [44]. This can be achieved directly by using vessel mimicking structures using microfabrication techniques such as 3D printing [49]. Moreover, the scaffold will need to support co-culturing of different cell types and also guide cell differentiation in a spatial manner. This can be achieved through anchoring nutrients [50] and inherent biomechanical properties e.g. elasticity, which has been shown to play a role in skeletal muscle development [51]. Furthermore, the scaffold should be edible and safe for human consumption if it is to be included in the final clean meat product. Hydrogels are being demonstrated as the most promising source of scaffold. They can be dynamic due to their ability to change shape based on light or potential signals, can be made from edible materials e.g. pectin and can also have nano-scale incorporation of materials e.g. growth factors [50].
In addition, the upscaling of clean meat production is one of the toughest technological hurdles facing the industry. This is due to the cost of clean meat being too high for it to be introduced into food markets, even as a premium product, despite the cost of a burger dropping from $300,000 in 2013 to $11.36 in 2017 (about 10x the cost of a standard burger) [52, 29]. It is widely believed that the successful upscaling of production is required to bring down the cost of clean meat by another 10x. The crucial development that would allow this is the production and use of large-scale bioreactors. These are the containers in which cells will proliferate and then ultimately differentiate into meat. Although bioreactors have already been developed and used in other cell-based systems, e.g. vaccine development and fermentation, the complex process of clean meat production poses new technological challenges. This includes sophisticated nutrient recycling systems which aim to minimise waste, as well as monitoring systems that would be able to change the supply of nutrients based on the stage of production. Furthermore, the bioreactors will need to be designed to incorporate the scaffolds used to grow clean meat.
There are also considerable non-scientific obstacles before clean meat can become a viable consumer product. It is currently unclear which organisations would actually regulate clean meat production. In the United States, the safety and quality of conventional livestock meat is under the jurisdiction of the US Department of Agriculture (USDA), whereas cell cultures and biomedicine are regulated by the Food and Drug Agency (FDA) [53]. However, a recent meeting of the FDA asserted that regulatory processes surrounding clean meat would be firmly within their jurisdiction, despite an earlier drafting bill from the US House of Representatives asserting the same, but for the USDA [54].
Another obstacle may also be the divisions within the industry about what clean meat should actually be. Some of the most influential names in the industry, including Bruce Friedrich of The GFI think that it preferable not to deviate from the normal composition of meat and change its fat and protein content, from a consumer standpoint [55]. However, others such as the CEO of Memphis Meats, Uma Valeti, think that a precision-engineered product will enhance consumer uptake—for example low fat versions of clean meat for health-conscious buyers [55]. Although both versions of livestock meat currently exist, it is unclear which marketing tactic will be optimal for the adoption of clean meat.
Following from this, a market for clean meat must be created so that the scientific and technological investment is not in vain. A 2016 survey carried out on US consumers revealed that most of the participants were willing to try clean meat, but only around one third were ‘definitely or probably willing’ to eat clean meat regularly in place of farmed meat [56]. The survey notes that the positive attitudes towards clean meat arise from the potential environmental and public health benefits of product, while negative attitudes come from reservations about the feasibility of industrial scaling and overtones of the ‘unnaturalness’ of meat grown in the laboratory (although this last query is incorrect because clean meat will be grown in bioreactors in food factories) [56]. One strategy to overcome this bias might be to reframe the questions asked in such surveys, emphasising the unnaturalness of the livestock production process [57]. A preliminary study conducted by researchers at Faunalytics and The GFI employed this strategy of positive framing [57]. They found that 45% and 52% of participants would be willing to regularly eat clean meat, and replace conventional meat with clean meat respectively (up from the roughly 33% from the Wilks study) [57]. A notable consideration which will influence consumer perception of clean meat is product labelling, something which has seriously hampered the reputation of GM foods among the general public [58]. There are ongoing disputes concerning this between leaders of the clean meat industry and those of the livestock meat industry [59]. Several petitions prohibiting the labelling of clean meat products as ‘clean’ or ‘beef’ have been produced by industry trade groups such as the US Cattlemen’s Association and the National Cattlemen’s Beef Association [59]. In opposition to this, the GFI has issued statements in the media, citing that such prohibition on labelling would misrepresent the final product [60, 61]. Careful management of consumer perception through marketing will be a critical aspect of the successful adoption of clean meat.
Finally, we feel that one rarely discussed obstacle to clean meat adoption requires attention: the impact of conventional meat industries on economic factors such as employment. As it currently stands, the proportion of total agricultural GDP that livestock creates is between 20-50% [62]. Globally, livestock contributes to 40% of agricultural value, according to FAO data, and ‘support[s] the livelihoods…of almost 1.3 billion people’. Given this massive reliance on livestock farming for millions of people, the economic void left by a potential switch to semi or fully-automated meat production by clean meat companies represents a huge concern for those currently employed in the livestock industry. It is therefore surprising how little attention has been given to the potential systemic change that clean might cause. Proponents of clean meat such as the GFI will have to manage these legitimate concerns and their bearings on clean meat’s consumer perception, and wider public discussion will be needed to address the social, economic and political consequences of a disruption to animal agriculture.
Summary and our proposals for the future of clean meat
In summary, we believe that clean meat looks set to overcome some of the world’s greatest current issues, though significant challenges remain. The global issues include the provision of meat to the world’s growing wealthy population; the ethical considerations regarding the suffering of animals on factory farms; and the potential danger to human populations from factory farming practices such as zoonotic pathogen escape and emerging antibiotic resistance. The challenges to the field most prominently include, but are not limited to, technological hurdles of upscaling cellular growth, efficient growth in serum free media and the availability of scaffolds. Non-technical challenges like the public perception of clean meat and its legislation by governments may prove to be the biggest barriers.
In light of the current state of the clean meat field and its main obstacles, we consider that there are four key areas where there is room for improvement: increasing scientific collaboration; alternatives to serum for media should be more routinely used in cell culture; public engagement with clean meat research should be increased and a policy infrastructure governing the sale, distribution and regulation of clean meat for the relevant authorities should be brainstormed. We believe the first of these is important as developing clean meat requires work from a wide range of scientific disciplines such as tissue engineering, materials science, stem cell research and chemical engineering. This will be needed to take clean meat from the laboratory to industrial food factories and poses a challenge as these fields have not traditionally collaborated significantly in the past. Therefore, new avenues of information exchange and understanding continue to need to be formed by scientists from these areas. Following on from this, sustained research into the development of non-animal derived culture media will also be necessary for clean meat to be truly free of animal slaughter. Linked to the point above, this will firstly require interdisciplinary research to initially determine the specific molecules a growth medium requires and then mass produce them in industrial quantities. We believe that setting a goal to replace serum in all cell culture media will lead to the much more rapid development of serum free media than if the research is confined to specific areas of research, e.g. clean meat and stem cell culture.
Finally, increased public and authority engagement is a necessity to educate the public on the problems of current meat production techniques as well as ensuring the proper publicity and regulation of clean meat production. Without this there may be a chance that a sizeable market for clean meat will not exist at the right time, when in other circumstances there might have already been a willing consumer base—nurtured through careful marketing and tactful public education about the failures of factory farming and the viability of clean meat. Appropriate development of the clean meat industry also relies on the imposition of regulatory bodies and distribution networks poised to turn the product into a widely available commodity.
References
1. P. Kotecki, “The Sergey Brin-backed startup behind the world’s first lab-grown burger just got a boost in the race to transform the meat industry”. [Online]. Available: http://uk.businessinsider.com/sergey-brin-backed-startup-boost-race-transform-meat-industry-2018-7. [Accessed: 05-Sep-2018]
2. J. Bunge, “Cargill Invests in Startup That Grows ‘Clean Meat’ From Cells”. Wall Street Journal, Aug. 23, 2017.
3. C. Godfray et al., “Meat consumption, health, and the environment”. Science, vol. 361, Issue 6399, July 20, 2018.
4. M. K. Bennett, “Wheat in national diets.” Food Res. Inst. Stud. 18, 37–76 (1941).
5. T. M. Marteau, “Towards environmentally sustainable human behaviour: Targeting non-conscious and conscious processes for effective and acceptable policies.” Philos. Trans. A Math. Phys. Eng. Sci. 375, 20160371 (2017).
6. United Nations, Department of Economic and Social Affairs, Population Division (2017). “World Population Prospects: The 2017 Revision, Key Findings and Advance Tables.” Working Paper No. ESA/P/WP/248.
7. FAO, “World Livestock 2011. Livestock in food security.”. FAO publications, 2011.
8. M. J. Post, “Cultured meat from stem cells: Challenges and prospects”. Meat Science, vol 92, pp 297–301, 2012.
9. D. Tilman, C. Balzer, J. Hill, B. L. Befort, Global food demand and the sustainable intensification of agriculture. Proc. Natl. Acad. Sci. U.S.A. 108, 20260–20264 (2011). doi: 10.1073/pnas.1116437108; pmid: 22106295
10. H. Valin et al., The future of food demand: Understanding differences in global economic models. Agric. Econ-Blackwell 45, 51–67 (2014). doi: 10.1111/agec.12089
11. N. Alexandratos, J. Bruinsma, World Agriculture Towards 2030/ 2050. The 2012 Revision. ESA Working paper No. 12-03 (FAO, 2012)
12. Hartmann, D.L., A.M.G. Klein Tank, M. Rusticucci, L.V. Alexander, S. Brönnimann, Y. Charabi, F.J. Dentener, E.J. Dlugokencky, D.R. Easterling, A. Kaplan, B.J. Soden, P.W. Thorne, M. Wild and P.M. Zhai, 2013: “Observations: Atmosphere and Surface.” In: Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change [Stocker, T.F., D. Qin, G.-K. Plattner, M. Tignor, S.K. Allen, J. Boschung, A. Nauels, Y. Xia, V. Bex and P.M. Midgley (eds.)]. Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA.
13. F.P. O’Mara, “The significance of livestock as a contributor to global greenhouse gas emissions today and in the near future” Animal Feed Science and Technology 166–167 (2011) 7–15.
14. FAO, “Livestock’s long shadow—Environmental issues and options.”. FAO publications, 2006.
15. Mario Herrero, Stefan Wirsenius, Benjamin Henderson, Cyrille Rigolot, Philip Thornton, Petr Havlík, Imke de Boer, Pierre J. Gerber, “Livestock and the Environment: What Have We Learned in the Past Decade?”. Annual Review of Environment and Resources 2015 40:1, 177-202.
16. Herrero, Mario et al. “Biomass Use, Production, Feed Efficiencies, and Greenhouse Gas Emissions from Global Livestock Systems.” Proceedings of the National Academy of Sciences of the United States of America 110.52 (2013): 20888–20893. PMC. Web. 5 Sept. 2018.
17. Mesfin M. Mekonnen and Arjen Y. Hoekstra, “A Global Assessment of the Water Footprint of Farm Animal Products.” Ecosystems (2012) 15: 401–415
18. H. L. Tuomisto and M. Joost Teixeira de Mattos, “Environmental Impacts of Cultured Meat Production”, Environ. Sci. Technol. vol 45, pp 6117–6123, 2011.
19. Isha Datar, “Environmental Impacts of Cultured Meat”. [Online]. Available: https://www.new-harvest.org/environmental_impacts_of_cultured_meat. [Accessed: 28-Aug-2018].
20. Schenck, R., Huizenga, D. (Eds.), 2014. Proceedings of the 9th International Conference on Life Cycle Assessment in the Agri-Food Sector (LCA Food 2014), 8-10 October 2014, San Francisco, USA. ACLCA, Vashon, WA, USA.
21. Carolyn S. Mattick, Amy E. Landis, Braden R. Allenby, and Nicholas J. Genovese, “Anticipatory Life Cycle Analysis of In Vitro Biomass Cultivation for Cultured Meat Production in the United States”. Environ. Sci. Technol. 2015, 49, 11941−11949.
22. G. Guglielmi, “Are antibiotics turning livestock into superbug factories?”. Science Magazine, Sept. 28, 2017.
23. M. E. J. Woolhouse and S. Gowtage-Sequeria, “Host Range and Emerging and Reemerging Pathogens.” Emerging Infectious Diseases, vol 11(12), pp 1842–1847, 2005.
24. A. Wasley and J. Robbins, “One in four UK abattoirs fails to meet basic hygiene standards”. [Online]. Available: https://www.thebureauinvestigates.com/stories/2017-02-20/one-in-four-uk-slaughterhouses-fails-to-meet-basic-hygiene-standards. [Accessed: 01-Sep-2018]
25. A. Peters, “Lab-Grown Meat Is Getting Cheap Enough For Anyone To Buy,” Fast Company, 02-May-2018. [Online]. Available: https://www.fastcompany.com/40565582/lab-grown-meat-is-getting-cheap-enough-for-anyone-to-buy. [Accessed: 04-Sep-2018].
26. “Cell-Ag 101”, Cleanmeat.info, 2018. [Online]. Available: https://www.cleanmeat.info. [Accessed: 18- Jun- 2018].
27. Chal, J. and Pourquié, O. (2017). “Making muscle: skeletal myogenesis in vivo and in vitro.” Development, 144(12), pp.2104-2122.
28. I. Datar, “Possibilities for an In Vitro Meat Production System”. Innovative Food Science and Technology, Volume 11(1), pp 13-22, Jan, 2010. https://www.sciencedirect.com/journal/innovative-food-science-and-emerging-technologies/vol/11/issue/1. [Accessed: 18- Jun- 2018].
29. E. Swartz, “The Science Behind Lab-Grown Meat,” A Bit of Science. [Online]. Available: http://elliot-swartz.squarespace.com/science-related/invitromeat. [Accessed: 01-Sep-2018].
30. I. T. Kadim et al., “Cultured meat from muscle stem cells: A review of challenges and prospects”. Journal of Integrative Agriculture, vol 14(2), pp 222–233, 2015.
31. “Clean Meat’s Path to Commercialization,” The Good Food Institute, 07-Dec-2016. [Online]. Available: https://www.gfi.org/clean-meats-path-to-commercialization. [Accessed: 02-Sep-2018].
32. Hocquette J-F, Gondret F, Beaza E, Medale F, Jurie C, Pethwick D W. 2010. Intramuscular fat content in meat-producing animals: development, genetic, and nutritional control, and identification of putative markers. Animal, 4, 303–319
33. S. Harris and U. Valeti, “Waking Up Podcast #28 – Meat Without Misery,” Sam Harris, 2016. [Podcast]. Available: https://www.samharris.org/podcast/item/meat-without-murder/. [Accessed: 01-Sep-2018].
34. E. Cosgrove, “Scale is the Real Barrier for Lab-Grown Meat,” AgFunderNews, 10-Oct-2017. [Online]. Available: https://agfundernews.com/scale-real-barrier-cultured-meat.html. [Accessed: 02-Sep-2018].
35. C. Sorvino, “Tyson Invests In Lab-Grown Protein Startup Memphis Meats, Joining Bill Gates And Richard Branson,” Forbes, 30-Jan-2018. [Online]. Available: https://www.forbes.com/sites/chloesorvino/2018/01/29/exclusive-interview-tyson-invests-in-lab-grown-protein-startup-memphis-meats-joining-bill-gates-and-richard-branson/#281238dc3351. [Accessed: 01-Sep-2018].
36. “Sustainable Seafood, Without the Catch,” Finless Foods. [Online]. Available: http://finlessfoods.com/. [Accessed: 22-Apr-2018].
37. E. Cosgrove, “BREAKING: Finless Foods Raises $3.5m Seed Round to Culture Bluefin Tuna,” AgFunderNews, 20-Jun-2018. [Online]. Available: https://agfundernews.com/finless-foods-raises-seed-culture-bluefin-tuna.html. [Accessed: 04-Sep-2018].
38. A. Kopelyan, “About,” IndieBio, 06-Feb-2018. [Online]. Available: https://indiebio.co/about/. [Accessed: 29-Apr-2018].
39. “The Good Food Institute,” The Good Food Institute, 31-Dec-2017. [Online]. Available: https://www.gfi.org/. [Accessed: 22-Apr-2018].
40. “2017 Year in Review,” The Good Food Institute, 02-May-2018. [Online]. Available: https://www.gfi.org/gfis-year-in-review-creating-the-good-food/. [Accessed: 25-Apr-2018].
41. “New Food Firm Created to Bring Clean Meat to China,” LIVEKINDLY, 30-Mar-2018. [Online]. Available: https://www.livekindly.co/new-food-firm-clean-meat-china/. [Accessed: 27-Aug-2018].
42. “Dao Ventures,” Dao Ventures. [Online]. Available: https://www.daoventures.com/. [Accessed: 28-Sep-2018].
43. “About | Oxford Martin Programme on the Future of Food | Programmes,” Oxford Martin School. [Online]. Available: https://www.oxfordmartin.ox.ac.uk/research/programmes/future-food/about. [Accessed: 22-Apr-2018].
44. E. A. Specht, “Opportunities for applying biomedical production and manufacturing methods to the development of the clean meat industry”. Biochemical Engineering Journal, vol 132, pp 161–168, 2018.
45. G. Gstraunthaler, T. Lindl, and J. V. D. Valk, “A plea to reduce or replace fetal bovine serum in cell culture media,” Cytotechnology, vol. 65, no. 5, pp. 791–793, 2013.
46. C.-Y. Fang, C.-C. Wu, C.-L. Fang, W.-Y. Chen, and C.-L. Chen, “Long-term growth comparison studies of FBS and FBS alternatives in six head and neck cell lines,” Plos One, vol. 12, no. 6, Jul. 2017.
47. M. E. Fernyhough, L. R. Bucci, J. Feliciano, and M. V. Dodson, “The Effect of Nutritional Supplements on Muscle-Derived Stem Cells in vitro,” International Journal of Stem Cells, vol. 3, no. 1, pp. 63–67, 2010.
48. N. J. Genovese, T. L. Domeier, B. P. V. L. Telugu, and R. M. Roberts, “Enhanced Development of Skeletal Myotubes from Porcine Induced Pluripotent Stem Cells,” Scientific Reports, vol. 7, no. 1, Jun. 2017.
49. J. Fu and D.-A. Wang, “In Situ Organ-Specific Vascularization in Tissue Engineering,” Trends in Biotechnology, vol. 36, no. 8, pp. 834–849, 2018.
50. M. P. Lutolf, P. M. Gilbert, and H. M. Blau, “Designing materials to direct stem-cell fate,” Nature, vol. 462, no. 7272, pp. 433–441, 2009.
51. P. M. Gilbert, K. L. Havenstrite, K. E. G. Magnusson, A. Sacco, N. A. Leonardi, P. Kraft, N. K. Nguyen, S. Thrun, M. P. Lutolf, and H. M. Blau, “Substrate Elasticity Regulates Skeletal Muscle Stem Cell Self-Renewal in Culture,” Science, vol. 329, no. 5995, pp. 1078–1081, 2010.
52. N. Shoemaker, “Price of Lab-Grown Burger Falls from $325K to $11.36,” Big Think, 29-Oct-2015. [Online]. Available: https://bigthink.com/ideafeed/answering-how-a-sausage-gets-made-will-be-more-complicated-in-2020. [Accessed: 28-Apr-2018].
53. E. Devitt, “Artificial chicken grown from cells gets a taste test — but who will regulate it?” Science Magazine, May 15, 2017.
54. Kelly Servick, “As lab-grown meat advances, U.S. lawmakers call for regulation. [Online] Available: http://www.sciencemag.org/news/2018/05/lab-grown-meat-advances-us-lawmakers-call-regulation. [Accessed: 05-Sep-2018].
55. R. Wiblin and B. Friedrich, “The 80,000 Hours Podcast with Rob Wiblin #20 – Bruce Friedrich makes the case that inventing outstanding meat replacements is the most effective way to help animals”, Rob Wiblin, 2018. [Podcast]. Available: https://80000hours.org/podcast/episodes/bruce-friedrich-good-food-institute/. [Accessed 07-Sept-2018].
56. M. Wilks and C. J. C. Phillips, “Attitudes to in vitro meat: A survey of potential consumers in the United States”. PLoS ONE, vol 12(2), Feb. 16, 2017.
57. Faunalytics and Good Food Institute, “Messages to Overcome Naturalness Concerns in Clean Meat Acceptance: Primary Findings. [Online]. Available: https://faunalytics.org/wp-content/uploads/2018/08/Clean-Meat-Acceptance-Primary-Findings.pdf. [Accessed: 05-Sep-2018].
58. J. Mohorcich, “What can the adoption of GM foods teach us about the adoption of other food technologies”. June 20, 2018. [Online]. Available: https://www.sentienceinstitute.org/gm-foods.
59. T. Agres, “Battle Brewing Over ‘Clean Meat’ Labeling”. [Online]. Available: https://www.foodqualityandsafety.com/article/clean-meat-labeling/3/?singlepage=1. [Accessed: 07-Sept-2018].
60. M. Ball, “GFI Fights Back Against Ill-considered Regulatory Mandates”. May 22, 2018. [Online]. Available: https://www.gfi.org/gfi-fights-back-against-ill-considered-regulator. [Accessed 07-Sept-2018].
61. The Associated Press, “Missouri considers nation’s first label for plant-based meat”. April 29, 2018. [Online]. Available: https://www.seattletimes.com/nation-world/missouri-considers-nations-first-label-for-plant-based-meat/. [Accessed: 07-Sept-2018].
62. FAO, [Online]. Available: http://www.fao.org/animal-production/en/. [Accessed 07-Sept-2018].

Meat without the animals: cleaning our conscience with clean meat by Alex Norman and Pranay Shah is licensed under a Creative Commons Attribution 4.0 International License.
<< Back to Contents
<< Back to Publications
St Anne's Academic Review (STAAR) A Publication by St Anne's College Middle Common Room ISSN 2048-2566 (Online) ISSN 2515-6527 (Print)
